Expanding into North America
Solvias Biologics and Novel Modalities Center of Excellence in North Carolina

Backed by a proven track record of supporting developers of advanced modalities across the full product lifecycle in Europe, Solvias is extending its expertise to meet the growing demand for GMP testing services in the U.S.
Our newest facility in Research Triangle Park, North Carolina, offers comprehensive support for biologics, cell therapies, gene therapies, and other novel modalities —from preclinical development through commercial release.
Our Biologics & Novel Modalities Hub in the US
Building on decades of expertise developed in Europe, creating a global network of world-class testing capabilities
We deliver a comprehensive portfolio of analytical services across therapeutic modalities and delivery systems, offering phase-appropriate solutions that streamline drug development and mitigate risks early on.
mRNA & Lipid Nanoparticles

Peptides & Oligos

Plasmids & Recombinant Protein
CRISPR
ADCs & mAbs
AAV & Lentivirus
Cell-Based Bioassays & Compendial Methods
Our RTP Center of Excellence is purpose-built to help clients overcome one of the most critical challenges in cell and gene therapy: developing robust cell-based bioassays.
- Potency Testing
- Immunogenicity Studies
- Antigenicity Studies
- Function Studies
- Mechanism of Action Studies
- Binding Assays
- Programed Cell Death
- Gene Expression
- Cell Proliferation
- Cell Signaling Assays
- Reporter Assays
- ELISA – UV vis, fluorescence, ultra-sensitive luminescence
- AlphaLISA
- Flow Cytometry – FACS Lyric
- Electrochemiluminescence – MSD
- Capillary Electrophoresis – Maurice CE
- Osmolarity (Ph.Eur. 2.2.35 USP<785>)
- Turbidity (Ph.Eur. 2.2.1; USP<855>)
- pH (Ph.Eur. 2.2.3; USP<791>)
- Extractable Volume (Ph.Eur. 2.9.17 USP<697/698>)
- Visible Particles (Ph.Eur. 2.9.20; USP<790>)
- Container Closure Integrity (dye ingress USP<1207>)
- Subvisible Particles (Ph.Eur. 2.9.19 USP<787/788>)
- Degree of Coloration (Ph.Eur. 2.2.2; USP<691>)
- Agilent 1260 HPLCw/ FLD & DAD
- Agilent 1290 HPLCw/ UV & FLD
- MACSQuant® Analyzer16 Flow Cytometer
- Orbitrap Exploris 240 Mass Spectrometer and Vanquish LC
- HIAC LiquidParticle Counter
- Illumina MiSeqi100+ System
- SCIEX PA 800+ CE-SDS, CGE, IVTRNA
- Maurice CE
- Maurice FlexcIEF & CE-SDS
From Vision to GMP-Ready in One Year
In roughly 12 months, we went from shell space to an operational laboratory to support complex therapies.
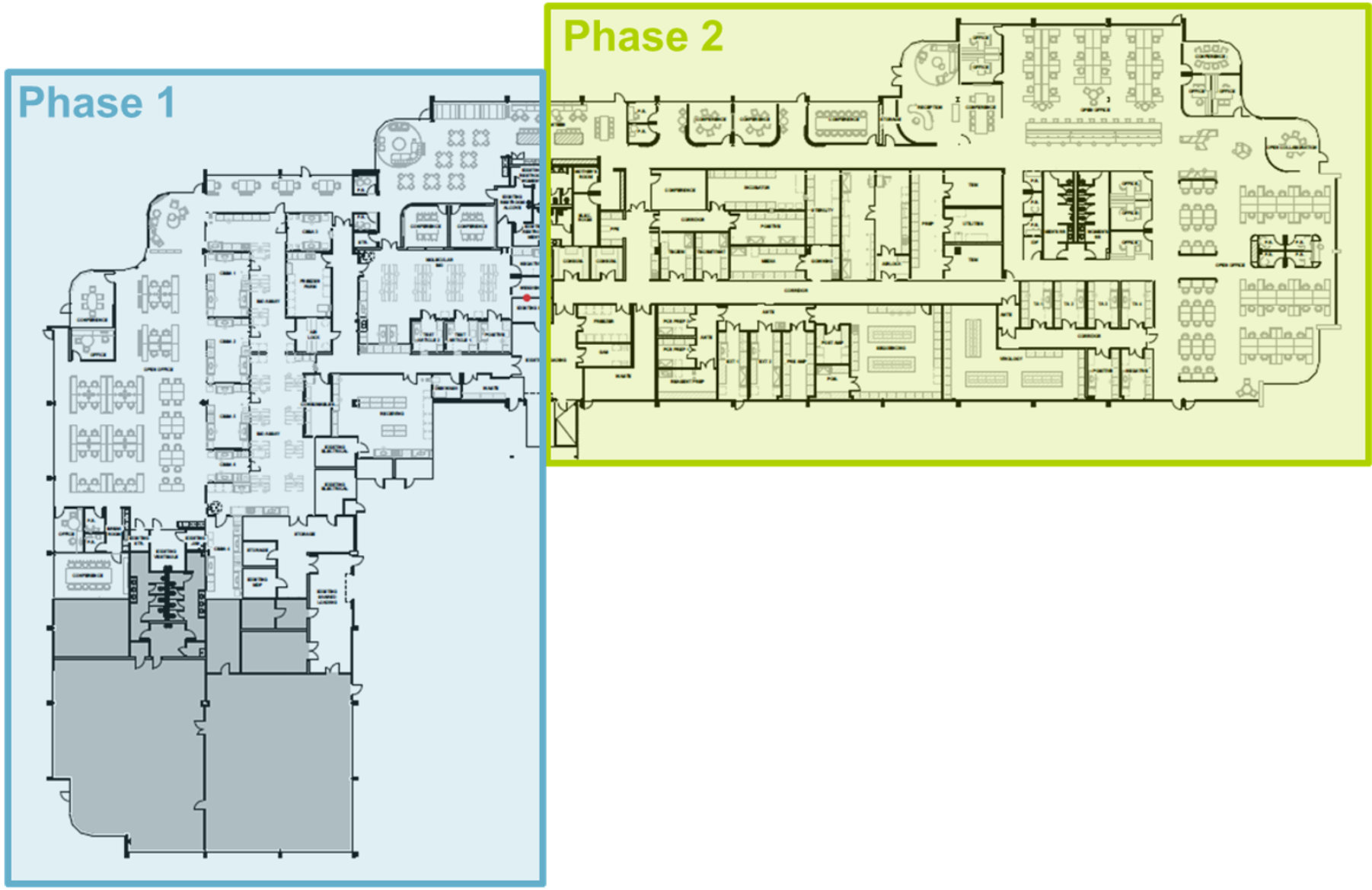

-
Phase 1: 20,000 sq ft with 6 cell-based bioassay suites & biophysical analytics labs, opened in January 2025.
-
Phase 2: 30,000 sq ft with the addition of molecular biology, virology and microbiology suites, opened in July 2025.
Our Growth Is Making Headlines
From industry journals to business news outlets, the media is highlighting Solvias’ expansion into the U.S. and the impact of our new RTP facility on the future of biologics, cell, and gene therapies development.










Downloadable Resources: Cell-Based Bioassays
To help you navigate the complexities of potency testing, regulatory requirements, and assay development, we’ve assembled a collection of expert cell-based bioassay resources, including webinars, white papers, e-books, and case studies. Explore real-world insights, proven strategies, and practical guidance from Solvias scientists to accelerate your development and de-risk your programs.
-1.png?width=1920&height=842&name=Untitled%20design%20(4)-1.png)


